Introduction To Graphene
Types Of Graphene
Properties of Graphene
Applications of Graphene
This Guide to Graphene Synthesis, Properties, and Applications is intended to convey a general understanding of these topics for both Scientists & Non-Scientists alike.
Introduction To Graphene
This introduction to graphene has been created to impart a general understanding of what graphene is, the types of graphene available, as well as synthesis methods and applications of graphene.
Graphene is quickly finding it’s way into a variety of applications and there are many advantages to using graphene to develop new products as well as to enhance specific properties in existing products.
What is Graphene?
Graphene is an allotrope of carbon that exists as a two-dimensional planar sheet. One way to think of graphene is as a single atomic graphite layer.
Graphene is technically a non-metal but is often referred to as a quasi-metal due to its properties being like that of a semi-conducting metal. As such, it has many unique properties that you don’t find with other non-metallic materials.
Each carbon atom is covalently bonded (sp2 hybridized) to three other carbon atoms in a hexagonal array, leaving one free electron per each carbon atom.
This free electron exists in a p-orbital that sits above the plane of the material. Each hexagon in the graphene sheet exhibits two pi-electrons, which are delocalized, allowing for an efficient conduction of electricity.
The holes in the structure also allow phonons to pass through unimpeded, which gives rise to a high thermal conductivity.
Graphene has many unique properties, making it an ideal material for use in electronic applications when compared to conventional materials.
Electrical conductivity the most prevalent and important property of graphene. Graphene doesn’t have an electronic band-gap (meaning that it can’t be switched on or off) as the valence and conduction bands have a small overlap and the electrons act as massless relativistic particles.
At room temperature, graphene can exhibit a concentration of charge carriers up to 1013 cm-2, with a mobility of 1 X 104 cm2 V-1 s-1. At low temperatures, this can increase to 2 X 105 cm2 V-1 s-1.
Because the charge carriers act as quasi-particles, otherwise known as massless Dirac Fermions, graphene also exhibits a half-integer Quantum Hall Effect (QHE).
The QHE is the relationship of the charge, density and velocity of the charge carriers. It occurs when a magnetic field is applied along the axis perpendicular to the plane of the conducting material.
Under these conditions, the path of the carriers becomes curved, leading to an accumulation of opposite charges at either end of the material. Due to the two-dimensional nature of graphene, the electron confinement produces discrete band levels known as Landau levels, which are filled by the charge carriers.
Unlike other materials, the charge carriers in graphene only half-fill these levels, leading to a quantization of the Landau levels, and in effect the energy levels of graphene.
Graphene also has great optical, thermal and mechanical properties. Single sheet graphene is a highly transparent material but each layer in thickness absorbs up to 2.3% of white light, with less than 0.1% reflectance.
There is also a linear absorbance increase with respect to the number of layers stacked on top of each other. A suspended graphene sheet can exhibit a thermal conductivity of 3000-5000 W m-1 K-1 at room temperature. However, this can drop to as low as 600 W m-1 K-1 when it is attached to another substrate.
The drop is caused by a scattering of phonons at the interface which impedes their movement, whereas in free standing graphene the phonon path is uninterrupted. Even at this lower conductivity, the thermal conductivity is still twice as high as copper.
Graphene is also known to be one of the strongest materials ever made and a single-layer graphene sheet can withstand up to 42 N m-1 of stress, with a Young’s modulus of 1.0 Tpa.
Types Of Graphene
There are many types of graphene. True Graphene is only one atomic layer thick (often called a monolayer) and it typically exists as a film but it can be floated off the substrate and can be redeposited onto another substrate or used in it’s isolated form.
There are, however, several types of graphene containing powder form materials such as graphene oxide, graphene nanoplatelets, graphene nanoribbons, and graphene quantum dots as well as graphene enabled products such as graphene ink or graphene masterbatches.
There are 3 main ways to synthesize graphene, they are:
- Chemical Vapor Deposition
- Chemical or Plasma Exfoliation from natural Graphite
- Mechanical cleavage from natural Graphite
Graphene can also be fully synthetic but those methods haven’t proven to be commercially viable.
Chemical Vapor Deposition (CVD) Graphene Films

Graphene films can be produced by varying methods, which include mechanical and thermal exfoliation, chemical reduction and epitaxial growth; but the most common method used in production today is by chemical vapor deposition (CVD).
CVD works by combining and depositing volatile gas molecules onto a substrate. The process takes place in a reaction chamber, where the material is formed on the surface of the substrate and the waste gases are pumped out.
Temperature dependence plays a vital role and can affect the type of reaction that occurs. CVD produces graphene films of high quality and purity, but the by-products produced during the reaction can be toxic due to the volatile nature of the precursor gases.
The graphene film is created by CVD in two steps. The first involves the pyrolysis of a precursor material to form carbon atoms on a substrate material.
By pyrolyzing the material on the substrate, carbon clusters are prevented from forming. Due to the amount of energy required to break the carbon bonds (C-C = 347 kjmol-1, C=C = 614 kjmol-1, C≡C = 839 kjmol-1, C-H = 413 kjmol-1), a high heat is required, and therefore a metal catalyst is required during the process.
The second step is a heat intensive step which assembles the dissociated carbon atoms onto a substrate (in the presence of a catalyst), which forms a single layer structure.
CVD graphene films are predicted to have strong chemical, electronic, mechanical and magnetic properties depending on the arrangement of the atoms in the film. Due to the five-order-of-magnitude difference between the grains and atoms at grain boundaries, only a few experiments have been produced to study these interactions.
One substrate that is known to produce high-quality graphene is copper. Copper acts as both a catalyst and the substrate.
The copper bonds to the carbon atoms, which provides strong carbon-substrate interactions, allowing for a single graphene layer to be easily formed on the surface. Copper oxide can also be inserted between layers of graphene, making it an easy process to remove a single layer.
Treating the copper substrate can also rearrange the surface morphology of the substrate-catalyst and is known to produce graphene with fewer defects.
Graphene Oxide (GO)
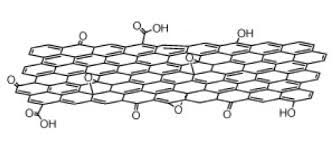
Graphene oxide (GO) is most commonly produced by the oxidation of graphite oxide. The oxidation process is beneficial, as it functionalizes the surface of the graphene layers with multiple species of oxygenated functional groups.
The multiple functional groups provide an enhanced layer separation and improved hydrophilicity. The hydrophilicity allows the graphene oxide to undergo ultrasonic irradiation, which produces a single/a few graphene layers that are highly stable when dispersed in DI Water and other solvents.
GO has many desirable properties. It disperses very easily in various mediums including aqueous solvents, organic solvents and various matrices.
The presence of both electron rich oxygen species and an electron rich graphene backbone allow for further surface functionalization, which gives rise to an adaptable material for multiple applications.
Graphene oxide does however suffer from a low electrical conductivity and is an electrical insulator. Graphene oxide is also soluble in many solvents, both aqueous and organic.

Graphene Oxide Is Highly Soluble
To gain the benefits of graphene oxide, it is typically dispersed, added into a formulation, made into a film or other nano-enabled product and then reduced to restore the graphene structure.
Reduced Graphene Oxide (rGO)
There are many methods to reduce graphene oxide (GO) into reduced graphene oxide (rGO), but most fall into three main categories: chemical reduction, thermal reduction and electrochemical reduction.
The other methods include hydrazine vapor treatment, annealing, laser and microwave reduction. The reduction process is vital to producing rGO, as it determines how consistent the rGO structure is with the GO precursor.
Many commercial producers of Graphene Nanoplatelets are in fact providing a product similar to industrial scale rGO as their GNP product. However this method differs from the rGO most people refer to which is a higher quality research product used for nano enabled devices.
Chemical reduction is a scalable method but can often result in poor yields and utilizes highly toxic materials such as hydrazine. rGO produced by this method generally exhibits a low surface area and has a low conductivity compared to the GO precursor.
Thermal reduction produces rGO with a high surface area that is close to the surface area of pristine graphene. However, the intense heating process causes a high-pressure build-up of carbon dioxide which causes structural damage to the graphene layers.
The structural imperfections can then give rise to a reduction in the overall mass (and yield against the theoretical output), vacancies, voids and it can hinder the mechanical strength of the material.
Electrochemical reduction shows the best results in terms of production and quality. The rGO produced is consistent with that of pristine graphene.
During the electrochemical process, the substrates (generally ITO or glass) are coated with a layer of GO and a current is passed through the material (via electrodes at either end of the substrate). rGO produced by this method have shown to have a high carbon to oxygen ratio and have exhibited conductivity comparable to that of silver.
The process also benefits from no toxic waste. This process does however suffer from issues regarding the feasibility of the scalability of the method.
Graphene Nanoplatelets (GNPs)

Graphene Nanoplatelets are typically synthesized by micromechanical cleavage of bulk graphite and can only produce graphene flakes in limited quantities which are mixed in with graphitic stacks. Large scale GNP production often uses mechanical cleavage followed by chemical reduction to produce the final GNP product.
Another method to make GNPs in bulk is by plasma exfoliation. A unique benefit of plasma exfoliation is the ability to synthesize and functionalize the GNPs to promote dispersion in the host matrix in a single, dry, processing step.
The RF or Microwave Plasma Reactor has a vacuum applied to remove atmospheric contaminants as well as residual contaminants which are liberated during the plasma milling process. These benefits make the material an excellent choice for large scale industrial applications with a wide variety of available functional groups such as OH, COOH, NH2, N2, & F.
Due to the lower cost of input materials, capital equipment, and plasma purification and/or functionalization, the GNPs can ultimately be a cheaper material than CNTs on a ton level thus paving the way for increased industrial applications with early adopters.
Graphene And Graphene Oxide Quantum Dots
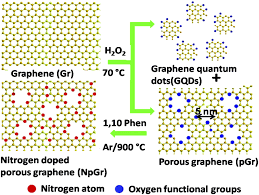
Graphene and graphene oxide quantum dots (GQDs) can be synthesized into various forms, from single-layer to tens of layers, but are generally less than 30 nm. GQDs also show similar properties to other types of quantum dots.
Like many graphene-based materials, GQDs exhibit a large surface area, a good linear dispersibilty and a high charge carrier mobility. GQDs also exhibit an efficient hole transporting ability, making them efficient materials for hole-transport layers.
They are useful materials for both electronic and opto-electronic applications.
GQDs can now be produced by a multitude of methods which includes both top-down and bottom-up approaches. Production by bottom-up methods can produce GQDs with a controlled size (due to the ability to control the band gap), but the synthesis itself can be complex which requires stringent conditions.
Top-down methods are in their infancy, but do provide a much simpler and cheaper approach. The breakdown of graphite oxide can produce single layered GQDs, but the yields aren’t high and the size distribution cannot be easily controlled. GQDs can also be synthesized by the breakdown of range of carbon allotropes including CNTs, fullerenes and carbon fibers.
Other methods include ultrasonication, microwave irradiation, radical methods and hydro-thermal or solvo-thermal methods.The main challenge that faces GQD production today is finding an efficient process that can take the production to industrial levels, as they have potential for many applications.
The applications of GQDs include lithium-ion batteries, supercapacitors and solar cells. But one area in which they are particularly useful is in LED displays. GQD-LEDs are a growing area and have been produced by etching CVD-grown graphene with block copolymers, followed by fabrication with graphite intercalating compounds.
The process produces graphene flakes that are low in oxygenated functional groups, of low toxicity and environmentally safe. It is also both a cheap and scalable process. GQDs emit light upon excitation, so are now being tested as emitters in organic light-emitting diodes (OLEDS). So far, they have exhibited a luminescence of 1000 cdm-2. The thin nature of GQDs may also open up future opportunities to create and perfect fold-able displays.
Graphene Nanoribbons
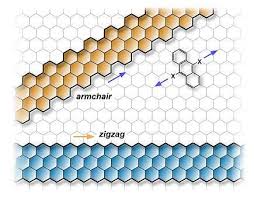
Unlike many other forms of graphene which are two-dimensional, graphene nanoribbons (GNRs) are quasi-one-dimensional materials with an ultra-thin width.
The electrical properties that GNRs exhibit are highly tunable and can be manipulated by dimension confinement, edge morphology and functionalization of the GNR.
GNRs also exhibit large aspect ratios, a high surface area, a conductive matrix and good mechanical flexibility. The combination of these properties has given GNRs a strong footing in composite materials for electronic applications. GNR anode composites have been shown to exhibit a specific reversible capacity of 1130 mAh g-1, with a coulombic efficiency >98%.
GNRs are produced by various methods. One of the most common methods involves unzipping the walls of MWCNTs with sodium and potassium-based compounds, sonicating and drying under vacuum.
They can also be produced by plasma etching of nanotubes onto polymer films, epitaxial growth and annealing on silicon carbide and CVD.
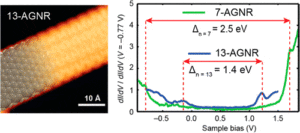
GNRs can enhance the performance of lithium-ion batteries through edge chirality effects. GNRs have a band gap that is inversely proportion to their width, which is dependent upon their edge chirality.
Chirality occurs at the edges because the electron confinement potential deforms the wave-function and causes the electrons move in a single direction, with more weight in the ‘positive x’ direction. This leads to a current in the ‘positive y’ direction.
For electronic applications, the edges in GNRs have shown the best results when armchair and metallic edges are present due to their semi-conducting abilities. Armchair edges also reduce the band gap energy when there is a defined width.
The energy at the edges of GNRs is proportional to their density and armchair edges are more tightly packed at the graphene interface, so the energy is higher than that of zig-zag edges. Such edges can be produced on GNRs by electron beam irradiation and electron beam lithography.
A GNR with a high concentration and purity of armchair edges has been found to provide highly efficient p-n junctions in electronic devices.
Graphene Aerogels

Carbon aerogels are derived by sol-gel synthesis methods and are a unique class of high-surface-area materials. Their high mass-specific surface area, electrical conductivity, environmental compatibility, and chemical inertness make them very promising materials for many energy related applications.
Recent developments in controlling their morphology make them especially well suited to super capacitor applications.
Aerogels are a special class of open-cell foams that exhibit many unique and interesting properties, such as low mass density, continuous porosity and high surface areas. These properties are derived from the aerogel microstructure, which consists of three-dimensional networks of interconnected nanometer-sized particles.
Aerogels are typically prepared by sol–gel methods, a process that transforms molecular precursors into highly cross-linked inorganic or organic gels that can then be dried using techniques such as supercritical drying, freeze drying, ect to preserve the insubstantial solid network.
For organic and carbon aerogels, the transformation involves the polymerization of multi-functional organic species into three-dimensional polymer networks.
Graphene Masterbatches

Graphene masterbatches are composite materials that contain a graphene-based compound (most commonly GO) and a polymer.
The graphene is used to enhance the properties of various common polymeric materials. Many polymers exhibit desirable properties such as low cost, low toxicity, bio-compatibility and chemical resistance, but they lack desirable mechanical properties.
By incorporating graphene nanoplatelets into polymer matrices, the polymers retain their original properties but benefit from enhanced rigidity and stiffness, while still being lightweight.
Using graphene as a filler compound rather than conventional inorganic materials can bring an enhanced electrical conductivity to the polymer, but it does have some issues.
In many graphene-based composites, graphene oxide acts as the dispersing support for other ions and molecules.
In polymer masterbatches, this can lead to problems as graphene doesn’t always disperse well in polymer phases (especially polyolefins) due to a lack of positive interactions at the grpahene polymer interface.
However, this can be overcome by the use of a surfactant, or by tailoring surface functionality of the graphene surface. The surfactant increases the surface interaction between the polymer and graphene.
If functionalized, the functional groups promote interaction between itself and the polymer molecules. If the functional groups aren’t compatible, you may observe what we call “islands of masterbatch” with easily observed islands of polymer in between well dispersed graphene-polymer masterbatches.
Properties Of Graphene
The properties of graphene are unique due to its all carbon structure and nanoscale geometry.
Electronic Properties

Because graphene has a delocalized pi-electron system across the entirety of its surface, the movement of electrons is very fluid.
The graphene system also exhibits no band gap, due to overlapped pi-electrons, allowing for an easy movement of electrons without the need to input energy into the system.
The electronic mobility of graphene is very high and the electrons act like photons, with respect to their movement capabilities.
The electrons are also able to move sub-micrometer distances without scattering. From tests done to date the electron mobility has found to be in excess of 15,000 cm2V-1s-1, with the potential of producing up to 200,000 cm2V-1s-1.
Thermal Properties
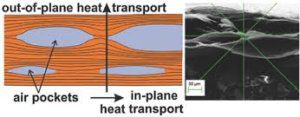
The repeating structure of graphene makes it an ideal material to conduct heat in plane. Interplane conductivity is problematic and typically other nanomaterials such as CNTs are added to boost interplane conductivity.
The regular structure allows the movement of phonons through the material without impediment at any point along the surface. Graphene can exhibit two types of thermal conductivity- in-plane and inter-plane.
The in-plane conductivity of a single-layered sheet is 3000-5000 W m-1 K-1, but the cross-plane conductivity can be as low as 6 W m-1 K-1, due to the weak inter-plane van der Waals forces.
The specific heat capacity for graphene has never been directly measured, but the specific heat of the electronic gas in graphene has been estimated to be around 2.6 μ J g-1 K-1 at 5 K.
Mechanical Strength
Graphene is one of the strongest materials ever discovered with a tensile strength of 1.3 x 1011 Pa. In addition to having an unrivaled strength, it is also very lightweight (0.77 mgm-2).
The mechanical strength of graphene is unmatched and as such can significantly enhance strength in many composite materials.
Flexibility/Elasticity
The repeating sp2 hybridized backbone of graphene molecules allow for flexibility, as there is rotation around some of the bonds, whilst still providing enough rigidity and stability that the molecule can withstand changes in conformation and support other ions.
This is a very desirable property as there are not many molecules that can be flexible and supportive at the same time. In terms of its elasticity, graphene has found to have a spring constant between 1-5 Nm-1, with a Young’s modulus of 0.5 TPa.
Applications of Graphene
There are many applications of graphene because it’s a revolutionary material. It has many applications replacing conventional materials as well as the ability to support applications previously not possible before the advent of 2D materials. The applications of Graphene are truly endless and many are yet to be conceived of yet.
Sensors
The ideal sensor is able to detect minute changes in its surrounding environment. Due to the planar and consitent arrangement of atoms in a graphene sheet, every atom within the sheet is exposed to the surrounding environment.
This allows graphene to effectively detect changes in its surroundings at micrometer dimensions, providing a high degree of sensitivity.
Graphene is also able to detect individual events on a molecular level. Many of graphenes properties are beneficial in sensor applications; as such, graphene could be used in sensors in various fields including bio-sensors, diagnostics, field effect transistors, DNA sensors and gas sensors, to name a few.
Batteries
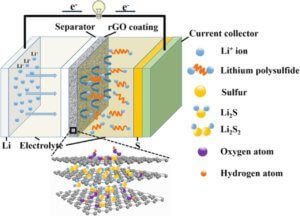
Graphene can be incorporated into both the anode or the cathode in various battery systems to increase the efficiency of the battery and improve the charge/discharge cycle rate.
The excellent electrical conductivity, surface area and dispersibility of graphene enhances the beneficial properties present in many traditional inorganic-based electrodes, whilst simultaneously relieving the electrodes of their limitations.
Due to its versatile nature, graphene has been incorporated into lithium-ion batteries, lithium-sulphur batteries, supercapacitors and fuel cells, of which there are multiple variations of each available on the market today.
Check out our detailed Graphene Batteries User’s Guide here.
Electron Emission Displays
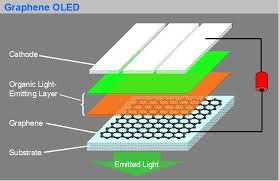
Graphene is an ideal material for use in electron emission displays as it exhibits a high aspect ratio and the dangling bonds at either end of the sheet allow for efficient electron tunneling.
The linear disperisty that the graphene surface provides produces massless Dirac Fermions. When exposed to an electric field, the field emission liberated electrons avoid all back-scattering because their escape velocity is independent to their energy.
Graphene can turn-on an electric field at 0.1 V µm-2, with a field enhancement factor of up to 3700. This can increase up to 4500 in screen printed graphene films.
Structural Composites
Graphene is incorporated into various composites for applications where strength and weight are limiting factors, for example in the aerospace industry.
Graphene is being incorporated into many materials to make the existing material stronger and more lightweight. For the aviation industry, a composite material which is much lighter than steel but will still provide the necessary strength will save a lot of money on fuel consumption, which is why graphene has started to be incorporated into such materials.
Graphene-based structural composites have a huge potential to become a widely used alternative to many materials used today.
Catalyst Supports
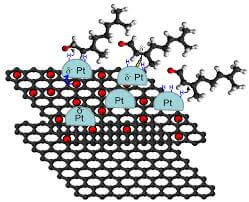
Even though the surface of graphene is planar and uniform, like any other material in existence it is subject to intrinsic defects.
Catalysts in the form of metal ions can sit in these cavities and be supported. In addition to providing mechanical support, the excellent charge carrier ability of graphene assists the charge transfer reactions involving the catalyst.
Graphene is also inert and does not interfere (in a negative way) with the interaction between the catalyst and the substrate materials. Graphene also provides an even dispersion of catalyst particles, so the catalyst-substate reaction is consistent across the whole support.
Polymer Masterbatches
Graphene can be incorporated into polymeric materials to form graphene-polymer composite materials.
As many polymeric materials suffer from strength-related problems, the incorporation of graphene can help to increase the tensile strength of the polymers, increasing the shelf life of the polymeric material in commercial applications.
Incorporating graphene into polymers can also give polymers electrical conductivity properties.
Functional Inks
Graphene can be used in functional inks for electronic, heat resistant and anti-corrosion purposes. By incorporating graphene into ink formulations, the conductivity properties associated with graphene influence the ink, causing it to become conductive.
The inks can then be used to coat electronics. Compared to other conducting inks, graphene is non-toxic, environmentally friendly, cheaper, quick-drying and recyclable. Graphene also has a high thermal stability, making it an ideal for heat resistant ink coating in electronic applications that produce large amounts of heat.
It is also an ink of choice when processing temperatures need to be high, as the graphene won’t break down during the manufacturing process. Graphene also exhibits excellent chemical stability and is inert.
For applications where environmental factors are an issue, graphene inks can provide a stable barrier to protect materials from chemicals and corrosion.
We hope you enjoyed this guide and found it informative. Graphene’s next killer app could be Your’s.
References:
Huang X., Xiaoying Q., Boey F. and Zhang H., Graphene based composites, Chem Soc. Rev., 2012, 41, 666-686
Zhou G., Yin L., Wang D. and Cheng H., A fibrous hybrid of graphene and sulfur nanocrystals for high performance lithium-sulfur batteries, ACS Nano, 2013, 7(6)
Cheng Q., Tang J., Zhang H., Graphene and carbon nanotube composite electrodes for supercapacitors with ultra-high energy density, Phys. Chem. Chem. Phys., 2011, 13, 17615-17624
Peng Z., Xiang C., Yan Z., Natelson D., Graphene Nanoribbon and Nanostructured SnO2 Composite Anodes for Lithium Ion Batteries, ACS Nano, 2013, 7(7)
Haegyeom K., Dong-Hwa S., Sung Wook None K., Kisuk K., Highly reversible Co3O4/graphene hybrid anode for lithium rechargeable batteries, Carbon, 2011, 49(1), 326-332
Bak S., Kim D., Lee H., Graphene quantum dots and their possible energy applications: A review, Current Applied Phyics, 2016, 11, 1192-1201
Liu Y., Dobrinksy A., Yakobson B. I., Graphene edge from armchair to zigzag: The origins of nanotube chirality, Phys. Rev. Lett., 2010, 105, 235502
Begliarbekov M., Sasaki K., Sul O., Yang E., Strauf S., Nano Lett., 2011, 11(11), 4874-4878
Pop E., Varshney V., Roy A., Thermal properties of graphene: Fundamentals and applications, MRS bulletin, 2012, 37, 1273-1281
Lei W., Li C., Cole M., Qu K., Ding S., Zhang Y., Warner J., Zhang X., Wang B., Milne W., A graphene -based large area surface-conduction electron emission display, Carbon, 2013, 56, 255-263
www.cheaptubes.com
www.graphenea.com
http://www.businesswire.com/news/home/20161101006012/en/Global-Graphene-Battery-Market-Worth-USD-115
http://s3.amazonaws.com/academia.edu.documents/41175514/Advanced_carbon_aerogels_for_energy_appl20160114-15050-1liyorj.pdf?AWSAccessKeyId=AKIAJ56TQJRTWSMTNPEA&Expires=1480256913&Signature=21XHgBv83B69AOLeWNHLpXxdIWs%3D&response-content-disposition=inline%3B%20filename%3DAdvanced_carbon_aerogels_for_energy_appl.pdf
http://www.4spepro.org/pdf/004401/004401.pdf
http://mceuengroup.lassp.cornell.edu/sites/mceuen/files/publications/JVSTB_Pushing_Graphene.pdf
https://arxiv.org/ftp/arxiv/papers/1301/1301.6181.pdf
http://www.graphene.manchester.ac.uk/explore/the-applications/sensors/
