Graphene Oxide
Unleash the Power of Graphene Oxide: Your Path to Exceptional Research Outcomes
Step into the exciting realm of nanotechnology with graphene oxide, a unique 2-dimensional array of carbon atoms that is highly oxidized and decorated with oxygen-containing functional groups. A marvel of nanotech design, graphene oxide’s unique solubility sets it apart, opening doors to a multitude of applications.
Our Offerings: Tailored Graphene Oxide Solutions
At Cheap Tubes, we provide a wide range of premium graphene oxide products designed to meet your specific research needs. Our offerings include single & few layer graphene oxide with varied platelet sizes, high oxygen content products freeze dried for enhanced solubility, and reduced graphene oxide (RGO). You can also choose from our graphene oxide gel and dispersions, perfect for a multitude of applications.
The versatility of our products makes them an excellent fit for various research and development scenarios. Each product is backed by our unwavering commitment to quality, ensuring you receive the very best in nanomaterial solutions.
Obtain Your Graphene Oxide Solutions Hassle-free
Our transparent pricing structure and easy-to-use platform allow you to quickly find the right graphene oxide product and get a quote instantly. Just select your desired product, enter the required quantity, and get an immediate quote.
For optimal longevity and performance, we recommend storing your graphene oxide in a lab refrigerator.
Ready to transform your research outcomes with our graphene oxide solutions? Obtain your quote online today. For further inquiries or tailored needs, don’t hesitate to reach out.
Explore More with Cheap Tubes
Your success in the nanotechnology realm is our shared victory. If you’re curious to discover more about how graphene oxide can supercharge your research, schedule a call with us. Together, let’s explore the possibilities and shape the future of nanotechnology. Your breakthroughs are the stepping stones to a future teeming with advanced nanotech solutions.”
Showing all 6 results
-
Few Layer Graphene Oxide 2-4L
$140.00 - $150.00 / per gram Select options -
Graphene Oxide Gel
$125.00 - $175.00 / per gram Select options -

Reduced Graphene Oxide
$190.00 - $200.00 / g Select options -
Single Layer Graphene Oxide
$140.00 - $150.00 / per gram Select options -
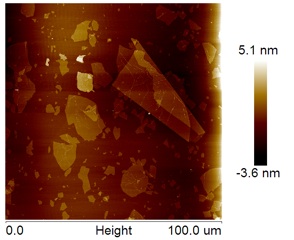
Single Layer Graphene Oxide 1-20um
$190.00 - $200.00 / per gram Select options -
Single Layer Graphene Oxide 450nm
$190.00 - $200.00 / per gram Select options
Properties
History
Synthesis
Applications
Structure
Molecular Weight
Reduced Graphene Oxide
Membranes
The Unique Properties of Graphene Oxide
Graphene oxide stands out owing to its unique properties like high surface area, functionality, and two-dimensional (2D) sheet-like structure. Its highly oxidized carbon atoms form a distinctive honeycomb hexagonal lattice pattern. The flakes range from nanometers to microns wide in the X and Y dimensions. The single-layer GO usually has a thickness of 0.7-1.2nm. The significant difference between graphite oxide and graphene oxide lies in the flake thickness. Materials thicker than 10 layers are generally classified as graphite, not graphene. The commonly available forms of GO include powder, solvent or polymer-dispersed form, or spin-coated film.

Graphene oxide can be easily dispersed in water, polymers, solvents, using ultrasonication or high shear methods like a homogenizer. Although GO is electrically insulating, it can be transformed into a conductive substance by reducing it, a process that removes most of the surface functionality and restores the graphene lattice structure
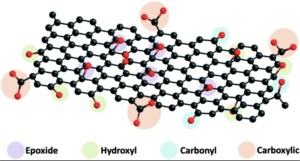
Understanding Graphene Oxide’s Physical Properties
Discussion about graphene oxide’s physical properties cannot omit its functionalization. GO usually possesses over 40% oxygen groups, including OH, COOH, and Epoxide groups. This unique physical property facilitates graphene oxide dispersion in Di water, NMP, DMF, THF, Ethanol, and other polar solvents. Fully oxidized, graphene oxide is a light brown (tan) solid powder with a C:O ratio between 2.1 and 2.9. Such properties are what give graphene oxide its excellent solubility and superior dispersion attributes.
The individual layers of GO retain the graphite structure but have a significantly larger and irregular spacing. The flake size usually ranges from hundreds of nanometers to tens of microns.
The Electronic, Optical and Thermal Characteristics of Graphene Oxide
Graphene oxide’s electronic properties are not as remarkable as pristine (CVD or Epitaxial graphene) graphene due to structural defects arising from oxidation, which disrupt the sp2 bonding networks. However, the conductivity of graphene oxide can be improved through reduction, which provides many benefits for real-world applications. It can also be combined with more conductive materials to enhance conductivity.
GO’s optical properties classify it as an optically transparent material. Although it’s not completely transparent, optical transparency greater than 90% can be achieved with GO films less than 5 layers thick. Each graphene layer reduces transparency by about 2%. Transparent conductive inks and films can be created by integrating GO with carbon nanotubes or silver nanowires.
Graphene’s superior thermal properties have triggered considerable interest, which is why graphene is widely studied for thermal management applications. Single layer graphene has high thermal conductivity, but data suggests that the addition of just one more layer significantly reduces its thermal conductivity. The interlayer spacing greatly affects thermal conductivity, implying that the layer count and the spacing between them can lower the overall thermal conductivity.
The thermal conductivity of graphene oxide surpasses that of bulk graphite with similar interlayer spacing. The increased interlayer spacing and the presence of oxygen groups amplify phonon scattering. Its high thermal conductivity can be attributed to enhanced interlayer coupling resulting from covalent interactions offered by the oxygen atoms
Graphene Oxide Thermal Conductivity
Research indicates that GO’s thermal conductivity correlates directly with the quantity of oxygen groups present. At 0.5% oxygen groups, the thermal conductivity of GO is about 50% less than pristine graphene. As the oxygen content escalates, its thermal conductivity drops. The minimum thermal conductivity of GO recorded in journal articles is roughly 8.8 W/mK, lower than the theoretical minimal thermal conductivity of 11.6 W/mK. This implies that we can customize the thermal transport in graphene oxide by managing oxidation and reduction.
Graphene Oxide’s Mechanical and Solution Processing Properties
The mechanical properties of graphene oxide are considerably lower than pristine graphene. Monolayer graphene oxide has a lower effective Young’s modulus (207.6 ~ 23.4 GPa when a thickness of 0.7 nm is considered) compared to “pristine” graphene, which has a Young’s modulus of about 1.0 TPa and the ultimate breaking strength of approximately 130 Gpa as reported by Lee et al. These properties are reduced due to the defects in the graphitic structure formed during oxidation. However, reducing GO can improve mechanical properties and restore the graphitic structure.
The high solubility of GO in many solvents and polymers facilitates the solution processing properties of graphene oxide. It can be spun, dipped, or coated onto a substrate using slot die, screen, gravure, or other printing methods. It can be patterned and reduced with a laser, even using a DVD writer in a PC has been shown.
Graphene Oxide History
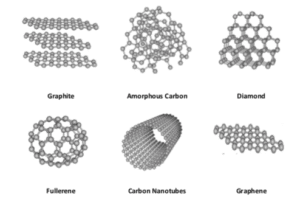
Graphene Oxide: A Journey Through Time Exploring Carbon Allotropes. Initially known as graphite oxide, the story of graphene oxide, spans over a century and a half. Oxford University’s chemist, Benjamin Brodie, was the first to produce it in 1859 using graphite flakes, potassium chlorate, and fuming nitric acid. A safer and quicker process called the Hummers method, developed by William Hummers and Richard Offeman in 1957, incorporated sulfuric acid, sodium nitrate, and potassium permanganate. Modern modifications of this method, such as the Tour method, prioritize environmental and safety precautions.
Synthesis Method

Synthesis of Graphene Oxide & Reduced Graphene Oxide Process Flow
A modified Hummer’s method synthesis method is typically used to make GO from graphite in chemical process by treating graphite with strong oxidizers such as potassium permanganate, hydrogen peroxide, sulphuric and hydrochloric acids.
This chemical process exfoliates the graphite into single or few atomic layer sheets, expands the interlayer structure, and adds the functional groups. The type and amount of oxygen containing functional groups makes graphene oxide hydrophilic which means that it’s water soluble.
Originally called graphite oxide, Graphene oxide synthesis involves reacting graphite with strong oxidizers, typically potassium permanganate and sulphuric acid, and is rinsed and centrifuged until the rinse water filtrate is PH neutral. Then it’s freeze dried to preserve solubility.
Some producers even use dialysis in the purification of GO. When GO is contaminated by alkaline salt byproducts generated during synthesis, it becomes highly flammable due to salt catalyzed carbon combustion.
Our GO is highly purified and flammability isn’t of concern. The bulk product is a brownish/yellowish solid material that retains the layer structure of graphite but with a much larger and irregular spacing.
There are four common graphene oxide synthesis methods: Staudenmaier, Hofmann, Brodie and Hummers. Variations of these methods exist, including the Tour method, a modified hummers method. It’s synthesis is constantly being studied and improved to provide a more consistent quality, reduce environmental concerns, and reduce costs. The oxidation process is typically evaluated by the carbon/oxygen ratio, >40% is considered acceptable.
Graphene Oxide Applications
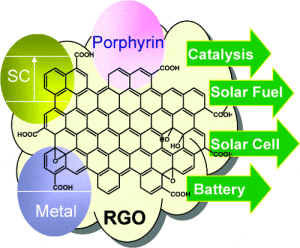
Graphene oxide applications include catalysis, drug delivery, solar, battery, tissue scaffolding, water desalination and many other areas. It’s high solubility and ability to be reduced enables solution processing, making it a desirable nanomaterial. GO overcomes the well known dispersion problems associated with other carbon nanotubes.
Electronic Applications
Electronic devices have been fabricated using GO as a starting material for at least one of the materials. Field effect transistors have been fabricated using RGO as well as chemical sensors and biosensors.
Transparent electrodes in the visible range of light are important for light emitting diodes (LEDs & OLEDs) and solar cell devices. In addition to transparent electrodes, RGO has been used as a hole transport layer.
Energy Storage Applications
RGO nanocomposites have been used for high capacity energy storage in lithium ion batteries. Electrically insulating metal oxide nanoparticles such as Fe3O4 or Fe2O3 can bond onto RGO and increase it’s performance in batteries. The energy storage capacity and cycle stability has been shown to increase when Fe3O4 is bonded onto RGO versus pure Fe3O4. High surface area GO has been synthesized using microwaves for exfoliation and can be reduced. The high surface area RGO is also used for energy storage material in supercapacitors.
Biomedical Applications
Studies have indicated that GO’s biocompatibility is good, paving the way for it’s use in drug delivery. It doesn’t induce oxidative stress because it’s preparation doesn’t involve metal catalysts and avoids metal impurities unlike other carbon nanomaterials such as carbon nanotubes (CNTa). The functional groups on the surface allow successful interaction with a wide range of organic and inorganic molecules by covalent, non-covalent (π-π or hydrophobic) and/or ionic interactions. This enables the use of GO for drug delivery applications.
Structure
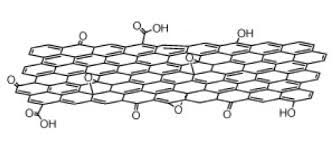
The 2D structure of Graphene oxide is shown above. The type and amount of functional groups present give GO it’s legendary solubility compared to other nanomaterials. Surfactants aren’t needed when dispersing into polar solvents such as Di Water, NMP, DMF, THF, DCB, Ethanol, polymers or others.
Molecular Weight
Graphene oxide’s chemical formula and molecular weight are below.
| Chemical Formula: | C140H42O20 |
| Molecular Weight: | 2043.856 g/mol |
Reduced Graphene Oxide
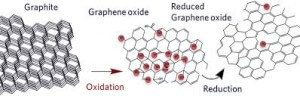
Reduced graphene oxide is synthesized as regular GO and then reduced. Reduction removes the surface functionality and restores the molecular structure to one closer to pristine graphene than GO.
Reduction is typically a chemical, thermal or electrochemical process. Other techniques are able to produce very high quality rGO, similar to pristine graphene, however it can be complex and time consuming to carry out.
Graphene Oxide Reduction Methods:
Treating with hydrazine hydrate and maintaining the solution at 100c for 24 hours
Exposing to hydrogen plasma
Exposing to a strong pulse light (photo sintering), such as xenon, UV, or laser
Heating in distilled water at varying degrees for different lengths of time
Heating to very high levels in a furnace
Directly heating in a microwave
Electrochemical methods
Heating to 400C in a forming gas atmosphere (95% argon, 5% hydrogen)
Chemical reduction is a highly scalable method but the surface area and electronic conductivity achieved typically isn’t high enough for many applications.
Heating GO to temperatures greater than 1000℃ creates RGO which has a very high surface area but the annealing process damages the structure when pressure builds up and carbon dioxide is released.The reduction process can reduce it’s mass by ~ 30% and improves its electronic properties.
Electrochemical reduction has been shown to produce very high quality RGO, almost identical in terms of structure to pristine graphene but can be slower than other methods.
RGO can be selectively functionalized, enhancing its compatibility with the solvent/matrix or to create new compounds when combining RGO with other two dimensional materials. It’s surface chemistry can be tailored for compatibility with the application.
Graphene Oxide Price
Graphene Oxide’s price is determined by production volumes and degree of purification. Most of products are between $75-200/g.
Our products are washed and centrifuged 15 times until the filtrate is pH neutral. Then it’s freeze dried to preserve solubility and stored in a refrigerator. Not all suppliers take these steps.
This process costs more than processes used by companies that sell GO in solution with a low pH. The low pH is because they’ve reduced the washing and centrifuging cycles and its an acidic solution.
GO’s shelf life is about 6 months before functionality begins to decrease however it can still be used longer. We recommend storing it in a lab refrigerator.
Difference Between Graphene & Graphene Oxide
First we must establish what is graphene or graphite. It is generally accepted that 2D sheets that are less than 10 layers thick can be called graphene.
There are 3 main types of graphene: Graphene films which are typically made by CVD, ALD, or Epitaxial growth, Graphene Oxide which is exfoliated by reacting graphite in sulphuric acid with potassium permanganate in a highly exothermic reaction, & Graphene Nanoplatelets which can be plasma exfoliated or chemically exfoliated, ball milled, or thermally shocked and sheared to product stacks of graphene nanoplatelets.
How to chose between graphene and graphene oxide? It depends on your application. If you want electronic properties to make a sensor or device or improved mechanical properties, we recommend CVD graphene, or RGO. If you want good dispersion and solution processing, we recommend normal GO.
Membranes
Graphene oxide membranes are relatively easy to make with vacuum or pressure filtration. GO’s known to disperse very easily due to the functional groups on its surface.
To make a GO membrane, first we disperse it in a solvent such as water or an organic solvent and then using a 0.2um membrane filter, pour the solution through a filtration apparatus, and the filter keeps the particles on top while the solvent is collected below forming the membrane on top of the filter.
When dry, the membrane can be removed leaving a free standing membrane paper like product.
Graphene Oxide Safety Data Sheet
Please see our Graphene Oxide Safety Data Sheet (SDS) GHS Compliant.
Graphene Oxide Dispersions
Dispersions are available from Cheap Tubes Inc. Please let us know your requirements such as solvent and loading ratio. Our standard concentration is 2mgs/ml but we can go up to 10mgs/ml.
Publications using GO from Cheap Tubes Inc
Below are some publications that used GO from Cheap Tubes Inc.
GO Membranes as ultrathin molecular sieves
Inkjet printed acrylic formulations using RGO
GO− Polyethylenimine Nanoconstruct as a Gene Delivery Vector and Bioimaging Tool
In situ Raman spectroelectrochemistry of GO
New RGO/Alumina (RGO/Al2O3) Nanocomposite: Innovative Method of Synthesis and Characterization
Scientists Create World’s Lightest 3D Printed Materials – Graphene Aerogel!
Scientists 3D print graphene-based inks for ultralight supercapacitors!
GO Elemental Analysis:
GO’s elemental analysis typically shows a higher oxygen content than carbon content with small amounts of hydrogen.
C: 35-42%
O: 45-55%
H: 3-5%
We can provide dispersions In Di Water, NMP, THF, or DMF. Hazmat shipping fees may apply.
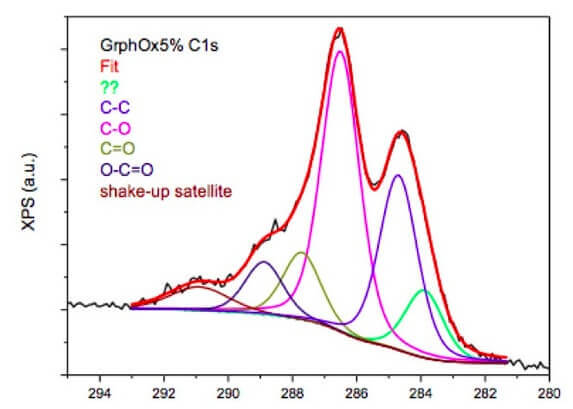
GO XPS data
XPS data is for our SLGO 300-800nm product is below
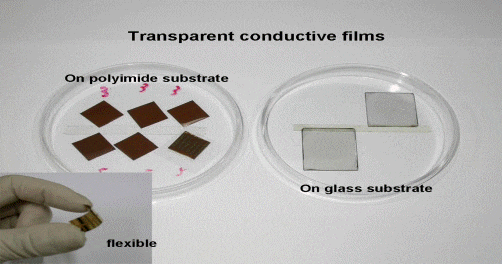
GO Films and Coatings
GO and RGO coatings are available with the specifications below.
- SLGO or SLRGO film on glass/wafer (thickness 5-30 nm, area ~ 3-5 cm2, conductivity 10(4) – 10(5) Sm-1, sheet resistance 10(1)-10(3) Ω/sq)
- SLGO or SLRGO film on organic flexible substrate (thickness 5-30 nm, area ~ 3-5 cm2, conductivity 10(3) – 10(4) Sm-1, sheet resistance 10(2)-10(4) Ω/sq)
Showing all 6 results
